This article is authored by Kriseira Lamas-Krauletz and Danny Rahal and is part of the 2020 pre-graduate spotlight series.
Background
Racial/ethnic minorities are more vulnerable to mental and physical health problems such as mood disorders, anxiety, and obesity. These differences in health outcomes may be due to stressful life circumstances, recent and long-term perceived discrimination, and/or socioeconomic status. To understand how these stressors can influence health and well-being, psychologists often study physiological hormones. By studying physiology, researchers can identify how psychological factors are influencing both the person’s psychological responses and the body’s physiological responses. Then, using both types of measures, researches can get a fuller picture as to how certain groups experience higher risk for poorer health over time.
One hormone that regulates a multitude of bodily functions is cortisol. You may have heard of cortisol in popular science and health articles because it is an important ’stress hormone.’ As the nickname implies, cortisol is reactive to stress and must reach homeostasis – a ‘sweet spot’ where the levels aren’t too high or too low – to keep your body functioning. It is involved in day-to-day functioning with other physiological systems. Physical increases in cortisol can have effects on other body systems (higher blood pressure, higher blood sugar, etc.) Cortisol can help control blood pressure and reduce inflammation, among other functions. The brain’s hypothalamus and pituitary gland sense if the blood contains the correct level of cortisol.
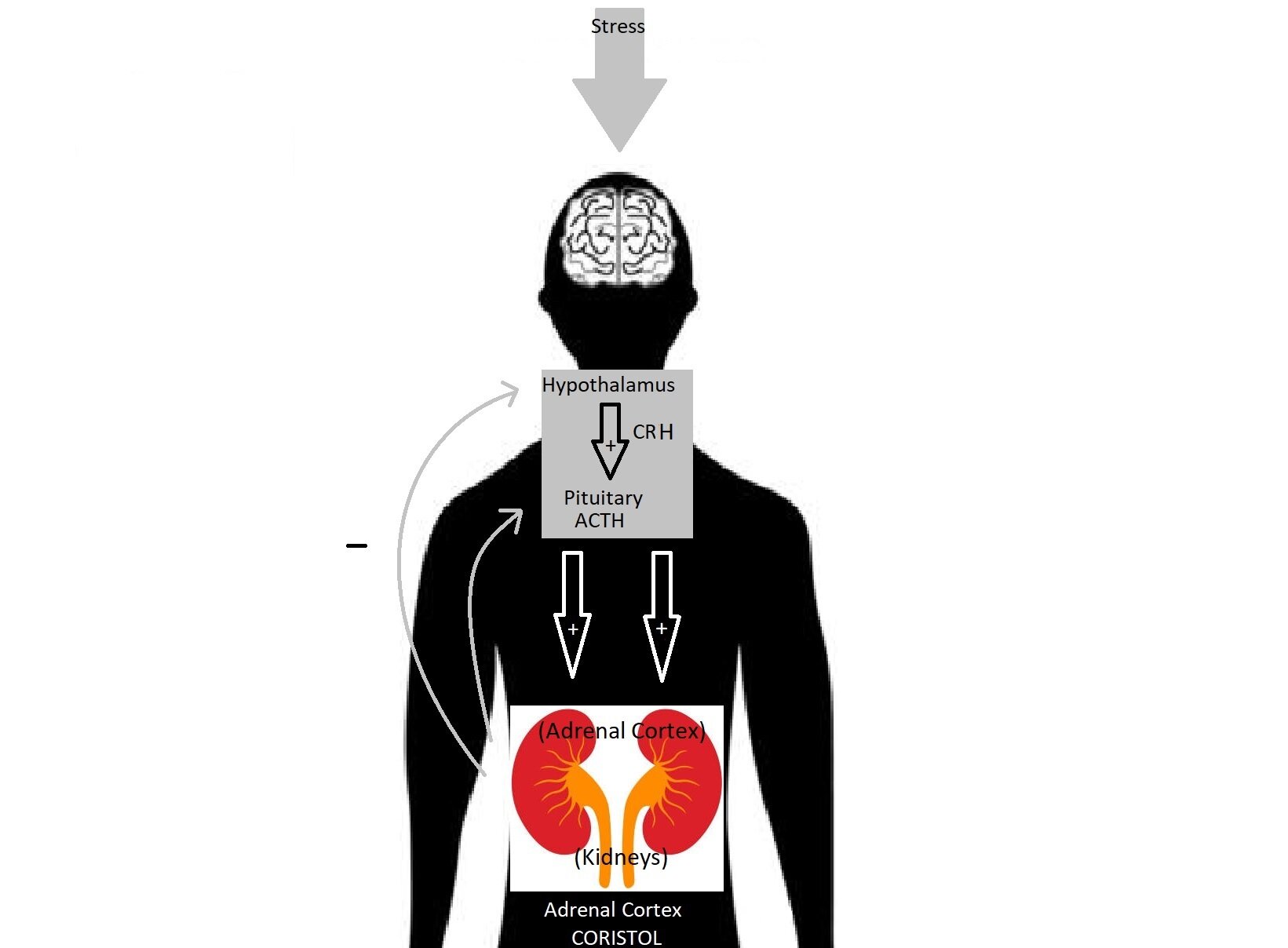
Cortisol is released as part of the hypothalamic–pituitary–adrenocortical (HPA) axis. When cortisol levels are off-balance, other parts of the HPA axis are able to modulate levels. Specifically, glands in the brain can signal the adrenal glands to increase or decrease cortisol release as needed. Psychologists love to measure cortisol levels because it’s an objective measure of the body’s levels of stress, whereas participant surveys on mood are subjective and not always reflected in bodily processes. In fact, it is very common for studies to measure both subjective reports of stress and physiological measures of stress such as cortisol, as both can be uniquely related to health.
A dysfunctional HPA axis results in a series of developmental cascades. Specifically, cortisol responds to stress and changes with the diurnal rhythm of cortisol, which is a biological rhythm that is synchronized with the day/night cycle. The diurnal rhythm of cortisol can be measure in a variety of ways. Changes in levels of cortisol from morning to evening are referred to as diurnal cortisol slopes. Previous research has shown that African American and Latino youth display flatter diurnal (daytime) cortisol slopes or greater daily cortisol output compared to White youth (DeSantis et al., 2007; Martin, Bruce, & Fisher, 2012; Zeiders, Doane, & Roosa, 2012). This is important because flatter diurnal cortisol slopes are associated with chronic stress.
HPA Axis and Cortisol Function
The HPA axis is the body’s stress response system that responds to stressors by activating cortisol secretion, especially when there is negative evaluation or rejection from others (Dickerson & Kemeny, 2004). The brain’s hypothalamus secretes corticotropin releasing hormone (CRH) into the bloodstream, which then signals the brain’s pituitary gland to secrete adrenocorticotropic hormone (ACTH). The secreted ACTH travels through the bloodstream to the adrenal cortex on top of the kidneys to signal the release of cortisol. When an adequate level of cortisol has been reached, CRH and ACTH stop being released. Previous studies have suggested that HPA-axis reactivity to stress is particularly high during adolescence post-puberty, translating to a higher secretion of cortisol (Klein & Romeo, 2013; McCormick & Zovkic, 2009; Sapolsky, Meaney, & McEwen, 1985; Vázquez & Akil, 1993).
Basal Functioning
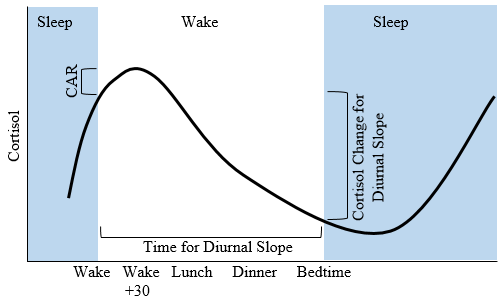
Cortisol has a diurnal rhythm and thus is a process with 24-hour oscillation. It is secreted across the day, with the highest levels 30 minutes after waking up and decreasing to its lowest levels at night, during sleep. These changes in cortisol levels throughout the day are linked with mental health and well-being. Because cortisol levels fluctuate throughout the day, psychologists look at different indices of cortisol to understand these changes throughout the 24-hour cycle: the cortisol awakening response, diurnal slope, and area under the curve. The cortisol awakening response (CAR) is the cortisol level difference between wake-up and 30 minutes post-wake-up (peak of cortisol levels), so it’s a response to waking specifically. A high CAR is associated with stress and negative anticipation for events in the morning (Dedovic & Ngiam, 2015). A low CAR is associated with PTSD, burnout, and systemic dysregulation (Speer, Upton, Semple, & Mckune, 2018). Thus, a CAR that is too low or too high is linked with poorer health outcomes.
The diurnal slope of cortisol, a negative slope, is the difference between the peak (30 minutes post waking) and rest (right before bedtime). Cortisol levels continue to decline throughout the night before rising again 30 minutes after waking up, where the cycle continues. The area under the curve (AUC) is the area under the time x log(cortisol) curve and it represents a measure of total daily cortisol output. A high AUC is associated with high stress levels (Saxbe, 2008).
The HPA axis is uniquely reactive to social-evaluative stressors. Not all stressors activate the HPA axis, and physiologist George Mason argued that characteristics of the individual (e.g., emotions) and of the stressors (e.g., unfamiliar or strange vs familiar stressors) can elicit a more helpless response. The characteristics that are more related to activation of the HPA axis response have been called Mason factors, which include uncontrollability, unpredictability, novelty, and ego involvement (e.g. how personal vs impersonal the situation is). For example, social evaluative threat is both uncontrollable and unpredictable and is a personal experience. When administering tasks, we need to ensure it is a novel experience. This may result in physiological (cortisol) or psychological (affect) stress responses. Consistent dysfunction of the HPA axis can interrupt diurnal cortisol functioning and lead to flatter diurnal slopes, as seen in studies with people of lower socioeconomic status.
An emerging area of research is if these developmental changes vary by race or ethnicity, so keep a look out for post 2!
References
Dedovic, K., & Ngiam, J. (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment, 11, 1181-1189. https://doi.org/10.2147/NDT.S62289
DeSantis, A. S., Adam, E. K., Doane, L. D., Mineka, S., Zinbarg, R. E., & Craske, M. G. (2007). Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health, 41, 3–13. https://doi.org/10.1016/j.jadohealth.2007.03.006
Dickerson, S., & Kemeny, M. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355-91. https://doi.org/10.1037/0033-2909.130.3.355
Klein, Z., & Romeo, R. (2013). Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Hormones and Behavior, 64(2), 357-363. https://doi.org/10.1016/j.yhbeh.2013.01.012
Martin, C. G., Bruce, J., & Fisher, P. A. (2012). Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones and Behavior, 61, 661–668. https://doi.org/10.1016/j.yhbeh.2012.02.025
McCormick, C. M., & Zovkic, I. Z. (2009). Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Progress in Neuro Psychopharmacology & Biological Psychiatry, 34, 756-65. https://doi.org/10.1016/j.pnpbp.2009.09.019
Sapolsky, R. M., Meaney, M. J., & McEwen, B. S. (1985). The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Developmental Brain Research, 18(1-2), 169-173. https://doi.org/10.1016/0165-3806(85)90261-5
Saxbe, D. (2008). A field (researcher’s) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review, 2, 163-190. https://doi.org/10.1080/17437190802530812
Speer, K., Upton, D., Semple, S., & Mckune, A. (2018). Systemic low-grade inflammation in post-traumatic stress disorder: A systematic review. Journal of Inflammation Research. 11, 111-121. https://doi.org/10.2147/JIR.S155903
Vázquez, D., & Akil, H. Pituitary-adrenal response to ether vapor in the weanling animal: Characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatric Research, 34, 646–653 (1993). https://doi.org/10.1203/00006450-199311000-00017
Zeiders, K. H., Doane, L. D., & Roosa, M. W. (2012). Perceived discrimination and diurnal cortisol: Examining relations among Mexican American adolescents. Hormones and Behavior, 61, 541–548. https://doi.org/10.1016/j.yhbeh.2012.01.018